CUWAA uses a web-based system, Cayuse, to submit and track submissions. Please click on the link at the bottom of this page to access Cayuse. You already have an account through CUWAA and just need to log in via Single Sign On to access it.
Once you are on the Cayuse landing page, click Products and then Human Ethics. You will see your researcher dashboard, where you can create new submissions, see the status of your projects, and access reviewer comments.
Researcher's instructions for Cayuse
Tips for an easy submission process:
- Cayuse is an interactive form, meaning new questions for your protocol will appear based on answers you select
- Download a copy of the submission template ahead of time to review what materials and answers you will need to know
- Save paragraph text in a Word document to save time by cutting and pasting
- Hit "save" frequently
- Set up a Cayuse Help Center account to find quick answers
Submitting a research application in Cayuse
Log in to the Concordia Portal > Click the Resource Tab > Find "Institutional Review Board (IRB) Human Ethics" the CAYUSE Image > Using your CUWAA single sign-on username and password, log in to Cayuse.
- Once logged in to Cayuse, click Products and then Human Ethics.
- Next, click on the blue "+ New Study" button in the upper right-hand corner. This will create a new folder.
- Enter the title of your study in the next box and then click on the blue check box.
- Click on the blue "+ New Submission" button in the upper right-hand corner, then select "Initial" This will begin a new study submission. You are automatically assigned as the PC (Primary Contact) for the study.
- Click on "Assign PI" to access the full submission. You can also access the full submission by clicking the "Edit" button in the study details page.
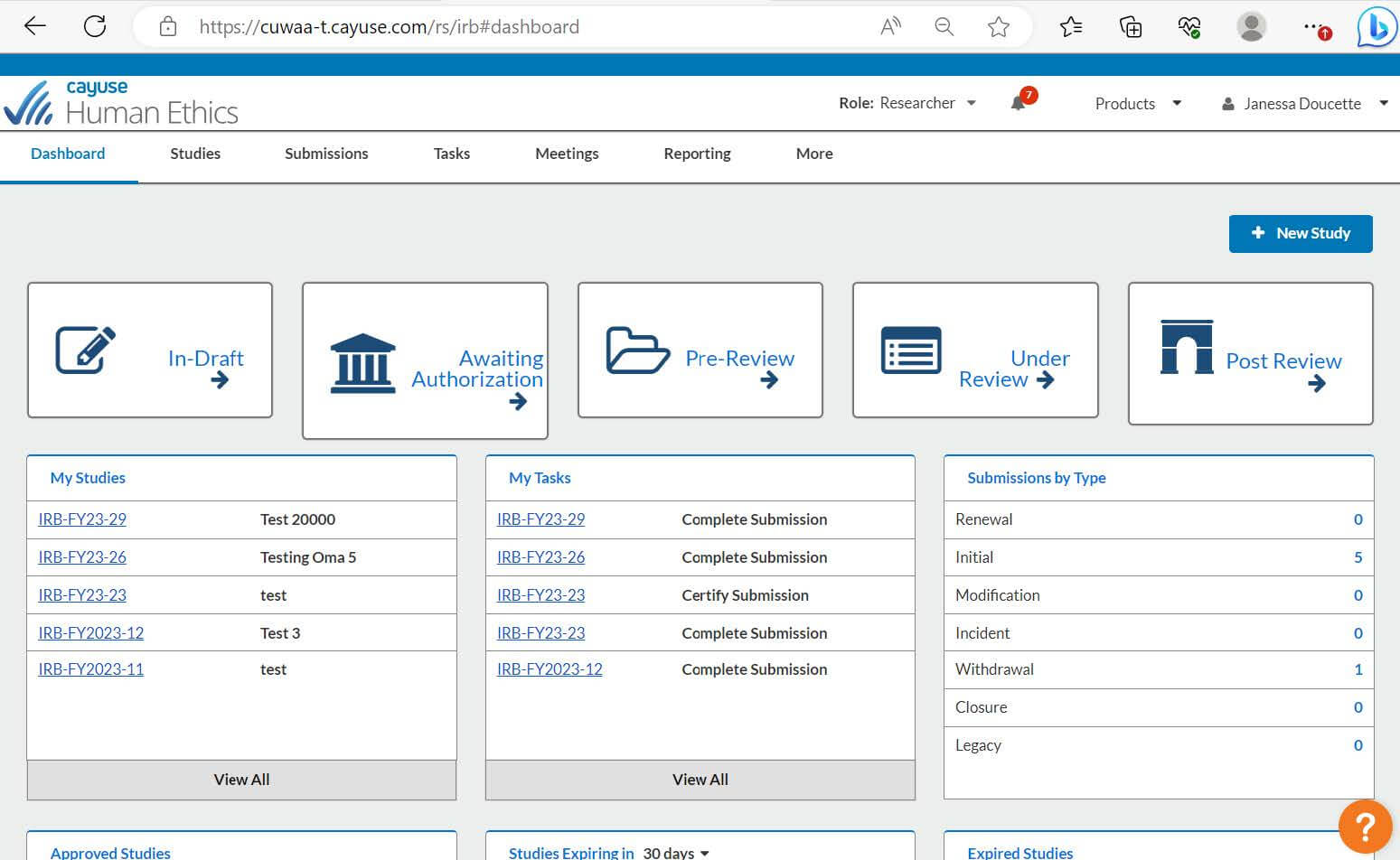
The landing page for Cayuse Human Ethics. Once you are logged in, you will be able to see all your studies, tasks, submissions, and more.
In this section you will review basic information about using Cayuse, find upcoming IRB committee meeting dates, and acknowledge that you have read the information. Select "Yes" and click on the forward arrow to continue.
- You will indicate what type of submission you are filling out. Most research at CUWAA will fall into the first category, Research Proposal. This includes theses, dissertations, and other kinds of research projects
- Next you will indicate whether your study is multi-institutional, meaning that it involves more than one institution. If you click "Yes", you will be asked a series of questions about the institutions involved in the study. If a different institution is the IRB of Record, you will be asked to attach documentation showing the required permissions
- Section 2 ends with a question about Study Category Determination. The category you select (Exempt, Expedited, Full Board) will prompt you to select a sub-category or provide more information about your study. If after reviewing all the options you are still not sure what category your research falls into, please contact the CUWAA IRB Administrator
Note: Exempt studies are reviewed by the IRB Administrator; Expedited studies are reviewed by the IRB Administrator and a minimum of two IRB Committee members; and Full Board studies are reviewed by the full IRB Committee.
- This section includes questions about your status at CUWAA, your study personnel, study site(s), and dates of research. You must select a Principal Investigator (PI), and if you are a student, you must also add your advisor as a Co-PI
- If you are conducting research in a campus classroom, you will be asked to read through a script that should be read to student participants before data collection. This acts as an informed consent process to help minimize coercion. You must agree to follow these requirements and read the script to students prior to data collection
- The last part of Section 3 is your research start and end dates. Remember that you cannot begin research until your submission is approved
You will indicate how many subjects you intend to enroll in the study, and their age range. Next, you will see a list of possible types of research participants and select all that apply.
- You will answer questions about your research project characteristics, including data collection methods, anonymity & confidentiality, whether you plan to present data in aggregate form
- The most important part of your application is also in Section 5: the Narrative Description. Here you will describe the purpose of your study, a justification and brief literature review, a concise description of methods, and a list of references
Note: Do not copy and paste large sections of text from a thesis, dissertation, or paper in lieu of answering the questions concisely.
- This section includes listing the person/people responsible for contacting subjects, your recruitment procedures, and copies of any advertisements or recruitment materials. You will also be asked to describe any incentives or compensation that participants may receive
- You will also be asked questions about Consent in Section 6, including being asked to upload copies of forms and language being used. If you are working with children, you will also be asked to upload a copy of an Assent form
- At the end of Section 6, you must agree to store your data for seven years in a secure location in accordance with CUWAA policy
- This section asks for further information on participant opportunities, as well as potential risks and expected benefits of participating in the research. You will be asked whether you intend to use deception as a method of gathering data, as well as how you plan to protect the identity of participants.
- You will answer questions about specific risks that research can entail and agree to the federal guidelines for handling those risks. You will also explain how you plan to handle any raw (identifiable) research materials.
This section is for disclosing any financial or other conflicts of interest that may exist between the research and PIs.
- You will attach your CITI training certificate in this section. This is mandatory for each personnel involved in the study
- The rest of this section will show what you have attached to the submission so far. You must make sure that all required documents are attached
Once you complete all the questions on the form, "Routing" and "Complete Submission" buttons will appear. If you cannot see it, please scroll to the very bottom of the blue section on the left. You will use the "Routing" button if you have a co-PI.
Note: if you are a student, your advisor must be listed as a co-PI in your application.
You must route your submission to your faculty advisor for approval before your application will be processed. You will be asked to Certify your application. Click "Certify" to finish your submission.
After you submit and your project personnel certify the application, it will be routed to the IRB Administrator’s queue or processing. Please note that the administrator handles sometimes dozens of submissions per day, and yours will be processed in the order in which it was received. If there are any issues with your protocol, the reviewer will leave comments for you to address within the protocol on Cayuse and you will receive an email that you need to look at them. Please address comments and questions thoroughly to avoid delaying your approval.
Answers to common Cayuse FAQs
On the righthand side of the screen, click Products and then Human Ethics. This will take you to your IRB Dashboard page.
Log in to Cayuse, click Products and then Human Ethics, and go to the box that says “My Tasks”. From there you can click into the protocol and certify it. Please do not certify protocols you have not read through.
Have everyone on your protocol log into their Cayuse account, click on their name in the upper righthand corner, and click My Profile. Then click Internal Associations. Make sure that at least one of your Internal Associations says “Primary Appointment”. If it does not, click on an appointment and toggle “Primary Appointment” to “Yes”.
Exempt and Administrative reviews take ten business days, if the protocol has no problems and is not missing anything. If your review is taking longer than that and you haven’t heard anything from the IRB, log into Cayuse and go to your dashboard to see if anything is in the My Tasks box. Often, someone on your protocol never certified it, and therefore it was never actually submitted. Sometimes reviews take extra time because of the volume we receive. The best thing you can do is submit early and plan well.
This happens often. Your reviewer sent it back to you/reopened it because there were mistakes or something was missing. Make sure to look closely at your protocol, section by section. If there is a reviewer comment, you will see a number next to the section title in Cayuse (click into your protocol, click Edit or Initial, and see the blue boxes on the left). That means you need to address their comment(s). Do not resubmit until you have addressed each one. You can respond directly to comments in Cayuse and should do so to ensure clear communication with your reviewer. After you resolve each comment and problem, resubmit your protocol. The ten-day processing clock starts over every time you resubmit, so take the time to make sure you are not missing anything the first time you submit.
If you have any questions, please contact IRBhelpdesk@cuw.edu.